The Precision
Oncology Company
About us
The Precision
Oncology Company
PANGAEA ONCOLOGY is a company focused on Precision Oncology, which offers both patients and pharmaceutical companies different services with the aim of improving the survival of cancer patients, their response to treatments, and their quality of life.
PANGAEA ONCOLOGY IN FIGURES
PATIENTS
VISITS
ACTS
TRIALS
CLIENTS
(FTE)
OUR CORE COMMITMENTS
PANGAEA ONCOLOGY’s mission is to change the natural history of the disease, improving the survival of cancer patients, through the application of the best professionals and technologies.
Position PANGAEA ONCOLOGY as the reference in Southern Europe in Precision Oncology, from patient treatment, the provision of services to the industry, the incorporation of disruptive technology and medical-scientific excellence, and directed towards systems intelligent IT and data exploitation (IA/BIG DATA).
OUR SERVICES
Molecular diagnostics
Rapid, accurate diagnosis. Our comprehensive & highly sensitive & specific range of tests can detect genetic alterations that drive tumor growth in all types of cancer.
DX platforms Validation
Molecular companion diagnostics tests provide essential information that enables doctors to identify those patients that are most likely to benefit from a treatment.
Pre-clinical services:
The PANGAEA LAB team is composed of prestigious research professionals and combines expertise in preclinical cancer research with extensive experience in R&D diagnostics.
our latest news & publications
- All Post
- Instituto Oncológico Rosell
- International Breast Cancer Center
- Pangaea Lab
- Pangaea Oncology
- Quénet Torrent Institute
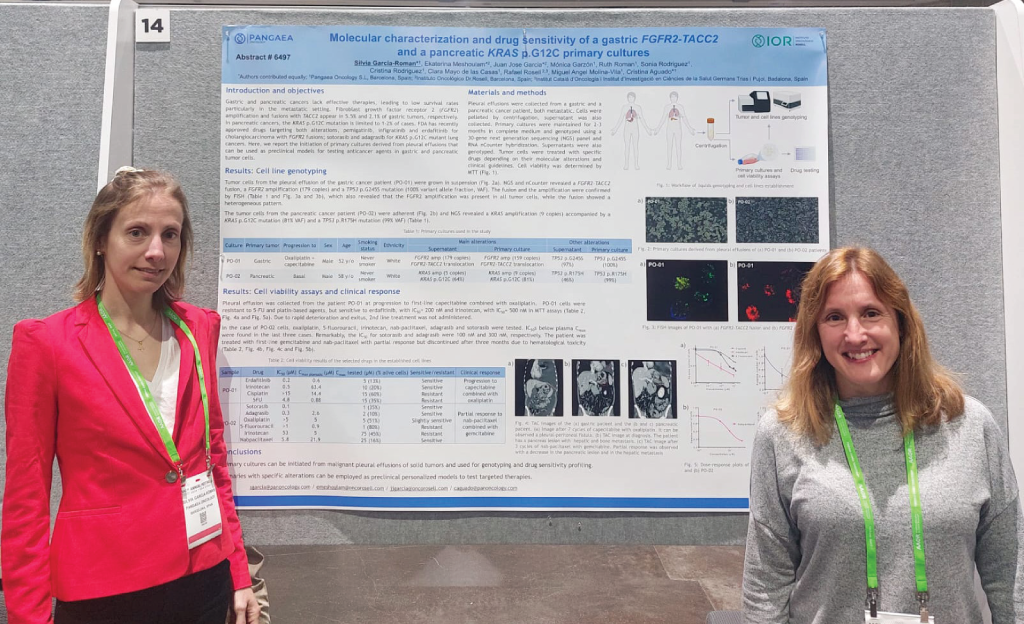
The study that demonstrates this has been presented at the AACR congress and is the result of the shared leadership of the scientists of Pangaea Lab, one of the centers with…

Rafael Rosell, world expert in lung cancer. "Avoiding adaptive resistance would be the beginning of controlling tumor clinical progression, since it would control acquired resistance. A challenge in lung cancer."

"Instituto Oncológico Dr. Rosell" and "NG Oncology" society, led by Dr. Joaquim Bellmunt, a renowned international oncologist in bladder cancer, announce their new joint collaboration at the Hospital Universitari Sagrat Cor…






